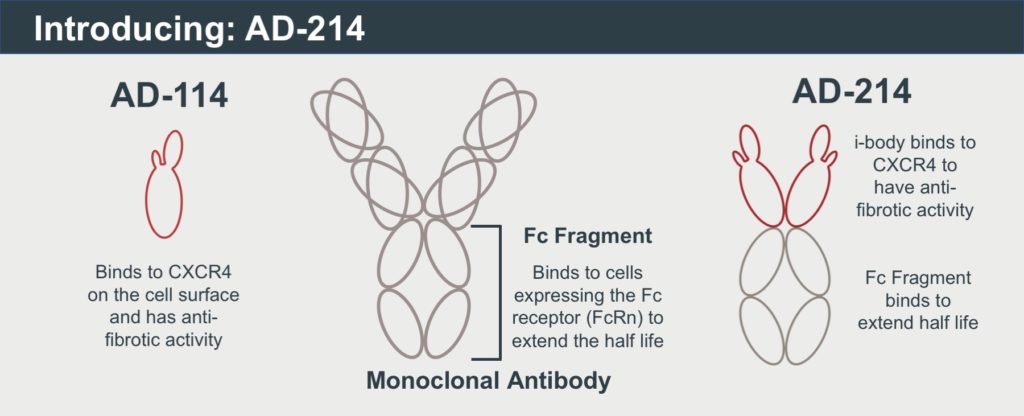
AdAlta, the Melbourne-based biotechnology company advancing AD-114 towards the clinic, today announces the publication of preclinical data in models of pulmonary fibrosis, generated in collaboration with the Cedars-Sinai Medical Centre in the US.
The publication titled ‘Anti-fibrotic Effects of CXCR4-Targeting i-body AD-114 in Preclinical Models of Pulmonary Fibrosis‘ appeared in Scientific Reports, an online, open access journal from the publishers of Nature.
In this pivotal pre-clinical dataset, researchers from AdAlta Ltd (La Trobe University, Australia), The Alfred Hospital (Melbourne, Australia) and Cedars-Sinai Institute (California, USA) show that the i-body AD-114 selectively targets and binds to CXCR4, a protein expressed at higher levels in patients with lung fibrosis. CXCR4 is believed to play a role in the recruitment of fibrotic cells to the lung, which is thought to contribute to the progression of lung fibrosis. Lung fibrosis, also referred to as idiopathic pulmonary fibrosis, or IPF, is a debilitating and life-limiting disease that causes irreversible scarring of lung tissue. The cause is unknown, and the scarring continues to worsen over time, making it difficult to breathe.
The prognosis of IPF is very poor with a median survival of only three to five years. Currently, there are just two treatments for IPF – Pirfenidone and Nintedanib – approved for use in Australia. These drugs are not curative but slow the disease progression, and patients tend to discontinue use due to severe side effects. AD-114 works on different pathways to both existing treatments for IPF.
La Trobe researcher and biochemist Dr Kate Griffiths, who was one of the first developers of the i-body technology and co-author of the paper, said:
“We are very excited with the research results, demonstrating that we can apply the i-body to an area of seriously unmet therapeutic need.”
“Our data add to the small but robust and growing body of literature showing that CXCR4 is an important alternative target for treating IPF and other fibrotic diseases. We have been able to show that the i-body AD-114 binds to lung tissue from IPF patients, and that the i-body blocks migration of some of the cells that are implicated in fibrosis without influencing or impacting the healthy cells. In an animal model, we have shown that the i-body has a protective effect on an artificially induced form of lung fibrosis.
“Unfortunately there is no cure for IPF as the scarring of the lung tissue is irreversible. With limited therapeutic options available to patients worldwide, there is a significant unmet medical need in the treatment of this rare lung disease. Our data however, clearly demonstrate the therapeutic potential of the i-body in the case of IPF and show strong promise as a future therapeutic option.”
AdAlta’s Chief Scientific Officer, Associate Professor Mick Foley, who also co-authored the paper and is an inventor of the i-body lead candidate AD-114, said:
“What is most remarkable about our i-bodies is their incredible specificity and affinity with their target, as these data show. Unlike existing treatments for IPF which have an unknown or very broad mode of action, the mechanism of action AD-114 is exquisitely specific and well understood and the new drug could potentially bring the progression of the disease to a grinding halt.”
AdAlta will be completing the final preclinical toxicology studies before moving AD-114 into the clinic, completing an initial first-in-human study in healthy volunteers in 2018.
Learn more about AD-114 and the platform technology from which AD-114 was developed, the i-body.