As part of an education series, AdAlta is explaining a range of debilitating diseases, including fibrosis. This post will focus on kidney fibrosis.
🔍 Let’s look at kidney fibrosis
Fibrosis is a condition where excessive scar tissue forms as a result of inflammation or damage, hindering normal functions. The kidney’s main job is to clean your blood, filtering any waste out through your urine. When your kidneys aren’t functioning properly, waste gradually builds up in your body, which can impact your health in many ways. This can lead to ‘chronic kidney disease’, where there is a loss of normal kidney function for more than three months.
Kidney fibrosis, also known as renal fibrosis, may be caused by chronic kidney disease or by the failed or incomplete healing of the kidney tissue after an acute injury. This causes the kidneys to stop working and eventually leads to the need for a transplant.
Kidney fibrosis occurs when there is an imbalance between the formation and degradation of the ‘extracellular matrix’, which is the network of proteins that provides support to the kidneys. As fibrosis progresses, the scar tissue replaces healthy kidney tissue, impairing the proper functioning of the kidneys.
Kidney fibrosis is a progressive process that ultimately leads to end-stage renal disease (ESRD). Treatments can include lifestyle and dietary changes at early stages, to keep blood pressure and blood glucose levels at safe levels. In later stages, treatments can include kidney transplantation or kidney replacement therapy (dialysis).
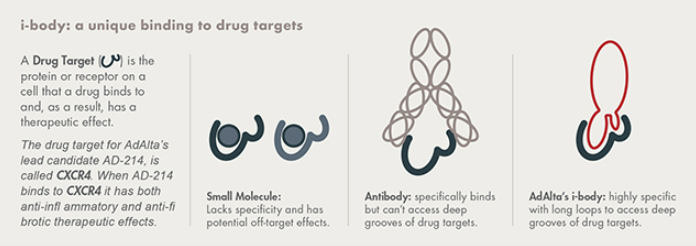
AdAlta has demonstrated that the lead candidate, AD-214, has broad anti-fibrotic effects in the lung, kidney and eye. As at October 2023, AD-214 is undergoing a Phase 1 extension clinical study, designed to assess the safety and availability of multiple 10 mg/kg intravenous doses of AD-214, which is the highest dose anticipated to be used in forthcoming Phase II clinical studies. We expect to report interim data by the end of 2023 and full data in early 2024.
Want to learn more? Visit the AdAlta Fibrosis page