
Update: Bill completes Long Kayak for Lungs
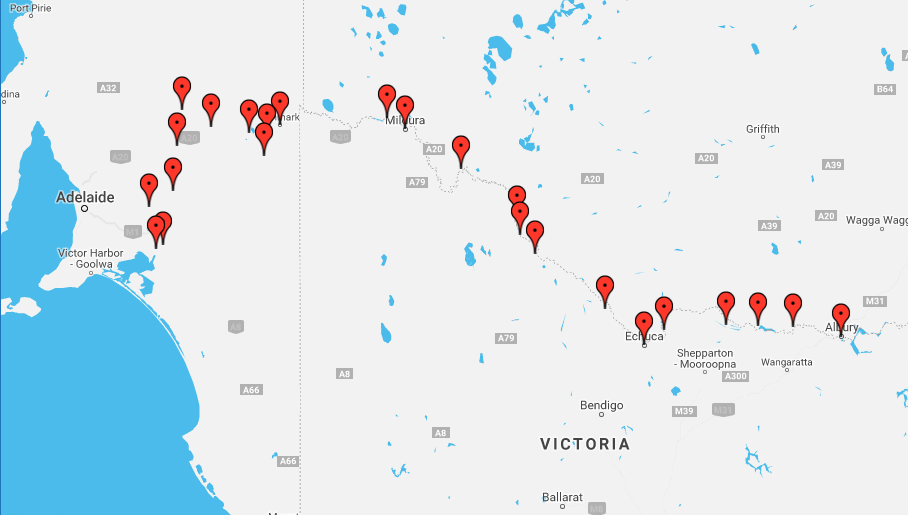
IPF patient Bill Van Nierop paddled into Wellington, South Australia yesterday to complete his 2,200km journey down the Murray River.
“The last 12kms was always going to be difficult mentally but more so as weather/river gods obviously decided to challenge to the end. After what seemed forever, at a time when Wal & I side by side in the river, Wal simply said, “you’ve done it, welcome to Wellington mate”.”
[Best_Wordpress_Gallery id=”11″ gal_title=”LKFL2″]
AdAlta is so proud of what Bill has accomplished, a herculean effort to tackle this challenge while living everyday with IPF. The strides he has made in raising, not only awareness of an often-neglected disease, but also $64,000 for the Lung Foundation are beyond remarkable. Learn more about Bill’s Long Kayak for Lungs and make a donation here.
At AdAlta we are focused on progressing AD-214, our potential treatment for Idiopathic Pulmonary Fibrosis (IPF). Our day-to-day activities are focused on the nitty gritty details of understanding fibrosis and how AD-214 works to reduce scarring of the lungs while also raising funding to continue our work.
In the Pulmonary Fibrosis Awareness Month of September we take a step back and focus on the patient behind the disease we’re working so hard to treat. Learn more about Pulmonary Fibrosis Awareness Month using #blueup4pf and #PFMonth.
Throughout September AdAlta will be following the journey of IPF patient Bill Van Nierop as he takes on the Long Kayak for Lungs, paddling 2200km over 42 days in a sea kayak down the Murray River. Bill, originally from Berri in Adelaide, grew up on the Murray River. Consider that IPF is characterised by difficulty breathing and you can understand how extraordinary a challenge Bill has set himself.
We were honoured when Bill reached out and asked for our support, last year Bill, 65, walked 770kms to raise awareness about IPF and funds for Lung Foundation Australia. Following his diagnosis of IPF, Bill has found little awareness of what Idiopathic Pulmonary Fibrosis is and that there is a stigma attached around the disease.
AdAlta is proud to support and promote Bill’s courageous journey; Bill’s story is a strong reminder of why we do what we do at AdAlta. Learn more about Bill’s Long Kayak For Lungs and make a donation.
Background
Bill was diagnosed with IPF in 2015. Over the last three years Bill has been on both of the existing treatments, initially Nintedanib and now Pirfenidone. Both treatments have had side effects, with nausea being common in both, and Nintedanib have additional side effects of sun sensitivity and diarrhoea.
Bill is looking ahead and hoping to be accepted on a clinical trial for new therapies in the near future. He is hopeful that AdAlta’s treatment AD-214 will one day be available to patients.

AdAlta visits at Echuca
On Sunday August 19, AdAlta’s CEO Sam Cobb and Chief Scientific Officer Mick Foley met Bill in Echuca, after he’d been paddling for about 10 days.

AdAlta CSO Mick Foley, IPF Patient Bill Van Nierop and AdAlta CEO Sam Cobb presenting a cheque from an AdAlta shareholder.
With only 50% lung capacity battling the effects of IPF including fatigue along with the side effects of treatment, Bill continues to push on despite the cold weather, whitecaps and strong head winds of 40kms recently seen in the Victorian region of the Murray.
Bill’s Experiences
On paddling with IPF: Really strong kayakers just power through this water, but effort required makes me breathless quickly so more stops. I need to find a different way of managing these patches.
On the battle with IPF: IPF battled me for lot of last 12-15kms…and it’s really a mental challenge, which I find much more tiring.
On the current treatment options for IPF: For those fellow IPF patients, they would understand that our medication like Pirfenidone, has some side effects, which we all live with. There are a lot worse off than me. However today I had to persevere with nausea & stomach cramps, first real day that there’s been an impact, so can’t complain.
On the side effects of IPF: Don’t mention it much, as part of my life now, but I’m susceptible to whatever causes nausea in my medications, & got hammered today.
On scale of 1-10, about 18, but usually being active gets me through it. Not today, had to live with it.
Related media
ABC News: Bill van Nierop to kayak 2200kms to raise awareness of IPF
Health Professional Radio: Long Kayak for Lungs 2018
Check Orphan: Australian lung fibrosis patient on long kayak for better treatments
PharmaDispatch: AdAlta backing Long Kayak for Lungs




